Initial C> Launches Faltered. The Next Wave Doesn’t Have to.
Four key considerations for commercializing the next wave of potentially curative therapies from Beghou Partner, Robert Rouse.
High costs, complex administration and slow uptake have complicated the sales ramp for the early wave of new gene therapies. Recent headlines notwithstanding, prospects for cell and gene therapy (C>) products remain bright.
But commercial execs need to rethink how they secure market access, urges Robert Rouse, a partner at Beghou Consulting.
“Much of the initial excitement around gene therapy has been tempered by reality,” explains Rouse, who guided one of the earliest CG&T launch teams toward success and presented at a May forum on pricing and reimbursement for these treatments.
That reality hit in the form of a slower-than-anticipated uptake curve. Questionable durability, for one, undermined the value proposition for some one-and-done medicines, keeping payers at arm’s length.
Lack of clarity on reimbursement, meanwhile, at times made providers wary of their sky-high price tags. And patients, in some cases adequately served by the standard of care, may have considered them a nice-to-have versus a necessity.
As a result, many in this first batch faltered. But the sluggish C> launch is a preventable occurrence. And Rouse is out to help biopharma avoid these well-known pitfalls going forward.
He offers a structured approach, derived from his experience launching hemophilia B treatment Hemgenix, that applies across the C> landscape.
Below are Rouse’s four key considerations that every aspiring brand should be able to answer before bringing such therapies to market:
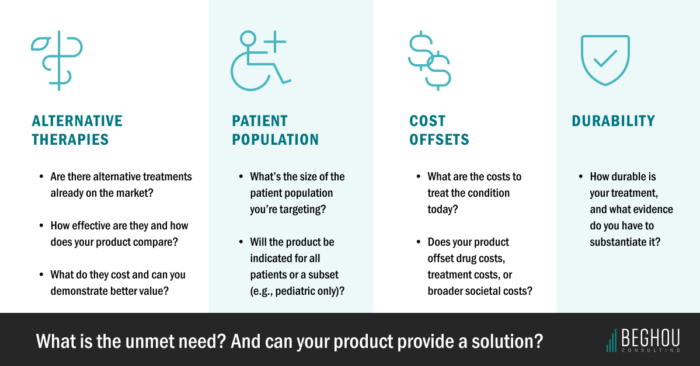
1. Consider the alternatives
Emerging C>s are usually poised to disrupt existing markets by displacing conventional treatments. But introducing the first potentially curative therapy in a category doesn’t automatically lead to widespread adoption. As previously mentioned, sometimes patients are well-served by the standard of care. That makes it more critical to identify the clinical gaps and demonstrate how an alternative can relieve economic burden by shifting real-world treatment patterns.
To that end, here are a few thought-starters:
- What outcomes do current therapies fail to achieve?
- What do those existing therapies cost annually and over a patient’s lifetime?
- How are therapies actually used in practice? Are patients adhering? Are providers managing care consistently?
Answering these questions helps clarify where your therapy creates differentiated value.
2. Narrow down the end user
Many C>s target populations in the hundreds, and it shapes trial design, value modeling, budget impact and payer engagement. Narrower populations (e.g., adult-only or patients who have failed other treatments versus broader categories) often mean more efficient targeting.
A couple of relevant questions:
- Does your clinical data align tightly with the real-world treatment population defined in the label, or are there evidence gaps?
- Have you pressure-tested your budget impact assumptions using payer-relevant inputs for population size, treatment dynamics and adoption curves?
You need trial data and cost modeling that directly reflect the labeled population, and your strategy must stay tightly aligned across functions to avoid gaps in access, education or evidence.
3. Where possible, tell the story through cost offsets
Gene therapies carry high upfront price tags, which means manufacturers must tell a compelling value story. The protagonist in this story? The litany of healthcare costs your therapy promises to eliminate or reduce over time.
Key considerations in this area:
- What direct costs are being eliminated?
- What clinical complications are being reduced?
- What data or models support cost claims?
Whether it’s long-term drug spending, hospitalizations or procedural burden, payers expect a quantified, evidence-based offset story.
4. Don’t discount durability
Durability can become the foundation for your pricing and reimbursement model. On the flip side, undercounting it could make payers do a double-take. They need assurances that covering the cost of that multimillion-dollar medicine won’t result in buyer’s remorse when patients relapse.
Solutions for helping ease this payer concern:
- Value-based contracts
- Amortized payment models
- Confidence in the therapy’s overall impact
That said, durability must be modeled transparently and supported with trial data or real-world evidence.
The aforementioned considerations are designed to add structure to a C> access strategy. However, Rouse cautions, they’re not meant as a mere check-the-box exercise.
Every aspiring next-gen product comes with its own unique science, story and constraints. But framing a strategy around these considerations can help uncover gaps, align teams internally and build a foundation for confident payer conversations.
“Locking in on all four points,” concludes Rouse, “will give you the best shot at a smoother launch.”

